|
Samples for chromatographic purification should be clear and free from particulate matter. Simple steps to clarify a sample
before beginning purification will avoid clogging the column, may reduce the need for stringent washing procedures and can
extend the life of the chromatographic medium.
Sample preparation techniques should ensure that components known to interfere with binding (the interaction between the
target molecule and the ligand) are removed. Since affinity chromatography is a binding technique, the sample volume does
not affect the separation as long as conditions are chosen to ensure that the target protein binds strongly to the ligand.
It may be necessary to test for a flow rate that gives the most efficient binding during sample application since this parameter
can vary according to the specific interaction between the target protein and the ligand and their concentrations.
The column must be pre-equilibrated in binding buffer before beginning sample application. For interactions with strong
affinity between the ligand and the target molecule that quickly reach equilibrium, samples can be applied at a high flow
rate. However, for interactions with weak affinity and/or slow equilibrium, a lower flow rate should be used. The optimal
flow rate to achieve efficient binding may vary according to the specific interaction and should be determined when necessary.
When working with very weak affinity interactions that are slow to reach equilibrium, it may be useful to stop the flow
after applying the sample to allow more time for the interaction to take place before continuing to wash the column. In some
cases, applying the sample in aliquots may be beneficial.
Elution
There is no generally applicable elution scheme for all affinity media. Reference to manufacturer's instructions, the scientific
literature and a few simple rules should result in an effective elution method that elutes the target protein in a concentrated
form. Elution methods may be either selective or non-selective, as shown below.
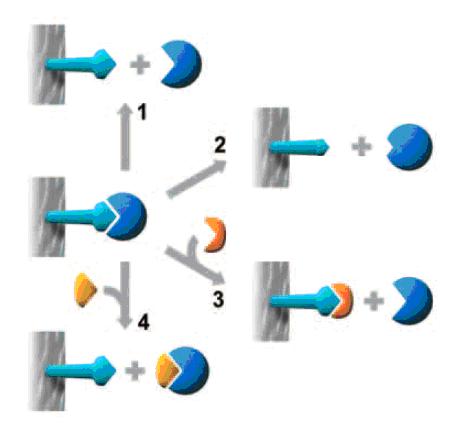
Method 1
The simplest case. A change of buffer composition elutes the bound substance without harming either it or the ligand.
Method 2
Extremes of pH or high concentrations of chaotropic agents are required for elution, but these may cause permanent or
temporary damage.
Methods 3 and 4
Specific elution by addition of a substance that competes for binding. These methods can enhance the specificity of media
that use group-specific ligands.
Flow Rates
It is not possible to specify a single optimal flow rate in affinity chromatography because dissociation rates of ligand/target
molecule interactions vary widely. For ready to use affinity media follow the manufacturer's instructions and optimize further
if required:
- determine the optimal flow rate to achieve efficient binding
- determine the optimal flow rate for elution to maximize recovery
- determine the maximum flow rate for column re-equilibration to minimize total run times
To obtain sharp elution curves and maximum recovery with minimum dilution of separated molecules, use the lowest acceptable
flow rate.
Analysis of Results and Further Steps
The analysis of results from the first separation can indicate if the purification needs to be improved to increase the yield,
achieve higher purity, speed up the separation or increase the amount of sample that can be processed in a single run.
It is generally recommended to follow any affinity step with a second technique, such as a high resolution gel filtration
to remove any aggregates, or ligands that may have leached from the medium. The chromatogram will also give an indication
of the homogeneity of the purified sample. Alternatively, a desalting column that gives low resolution, but high sample capacity,
can be used to quickly transfer the sample into storage buffer and remove excess salt.
Example in Practice
The goal of affinity chromatography is to separate all the molecules of a particular specificity from the whole gamut of molecules
in a mixture such as a blood serum. For example, the antibodies in a serum sample specific for a particular antigenic determinant
can be isolated by the use of affinity chromatography.
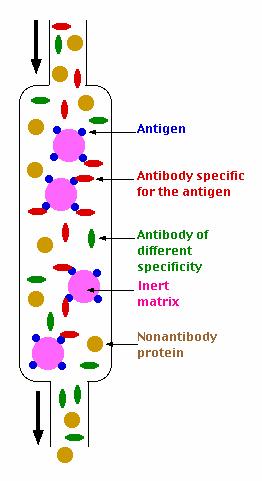
Step 1
An immunoadsorbent is prepared. This consists of a solid matrix to which the antigen (shown in blue) has been coupled
(usually covalently). Agarose, sephadex, derivatives of cellulose, or other polymers can be used as the matrix.
Step 2
The serum is passed over the immunoadsorbent. As long as the capacity of the column is not exceeded, those antibodies
in the mixture specific for the antigen (shown in red) will bind (noncovalently) and be retained. Antibodies of other specificities
(green) and other serum proteins (yellow) will pass through unimpeded.
Step 3
Elution. A reagent is passed into the column to release the antibodies from the immunoadsorbent. Buffers containing a
high concentration of salts and/or low pH are often used to disrupt the noncovalent interactions between antibodies and antigen.
A denaturing agent, such as 8 M urea, will also break the interaction by altering the configuration of the antigen-binding
site of the antibody molecule.
Another, gentler, approach is to elute with a soluble form of the antigen. These compete with the immunoadsorbent for
the antigen-binding sites of the antibodies and release the antibodies to the fluid phase.
Step 4
Dialysis. The eluate is then dialyzed against, for example, buffered saline in order to remove the reagent used for elution.
|


